
Authored by Rona Gungon, QTS Project Engineer
Since the implementation of the FDA Unique Device Identification (UDI) regulation, medical device manufacturers have been presented with the challenge of how to present the UDI symbols on their packaging.
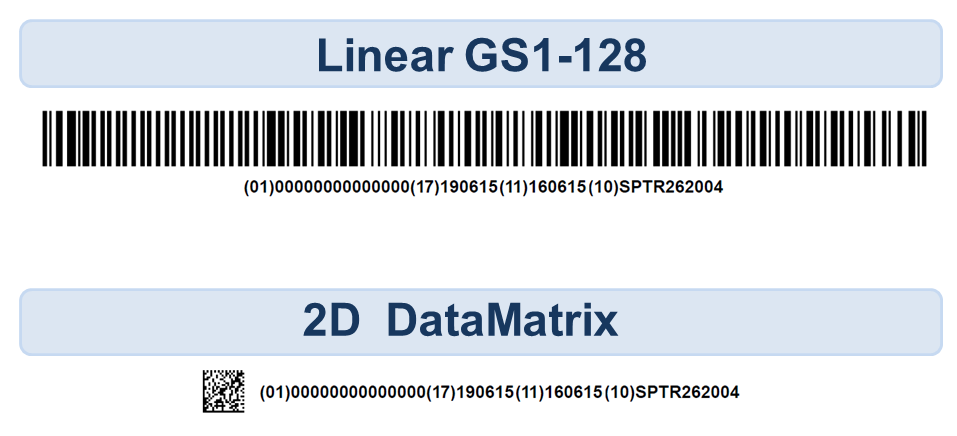
The size and shape of device packaging significantly impacts the available space for a symbol, and therefore the size of the symbol must be adjusted to suit. Depending on the data that needs to be encoded within the symbol, a traditional linear barcode like the GS1-128 can take up considerable space, as it grows wider as more data is encoded. A package large enough to accommodate a long linear barcode, such as a surgical kit, may not pose an issue. However, many medical devices are quite small and are often distributed in small packages (e.g. pedicle screws or dental implants). Fortunately, 2D barcodes are able to encode all of the required UDI data in a considerably smaller area than a linear symbol would typically occupy.
In the GS1 barcode examples shown below, the same UDI information (GTIN, Expiration Date, Manufacture Date and Lot Number) are encoded. Unless the package and label have the space to accommodate a long linear barcode, a 2D barcode may be more appropriate for the application.
QTS has the expertise and the experience to help our customers in choosing the right barcode for their packaging. If you have questions about UDI and medical device labeling, please contact your QTS account manager, or email us at expert@qtspackage.com.



