There are many ways to test your package or pouch for imperfections. Each method provides a unique output and can help identify crucial data for improving your finished package. We asked our Project Engineering Manager, Brian Nissen, to highlight 3 testing methods commonly used for medical device packaging.
1) Tensile Testing
Tensile testing is used to test the strength of the bond between the two layers of the package. Typically, you will cut a one-inch-wide strip from the sealed area of the package and place it in the tensile testing piece of equipment. Place one piece of the sample in the upper grip, and one in the lower grip. You will then record the tensile strength as that package sample is pulled.

Seal tensile testing produces variables data – it is a number of pounds per inch of seal width. This is great for measuring a process to see whether or not it remains within statistical control.
There are multiple methods of data capture (average strength, peak strength, etc.), which you can learn more about here.
2) Dye Penetration Testing
When performing a dye penetration test, you will open a small part of your package and inject a dye. This dye is primarily water with a small amount of powdered dye solution as well as a surfactant. The surfactant lowers the surface tension of the solution so that it will flow through any channels or imperfections.
Once the dye has been injected into your package, you will inspect it for any imperfections. If there is a channel within the seal of your package, you will see the dye running through it within a number of seconds.
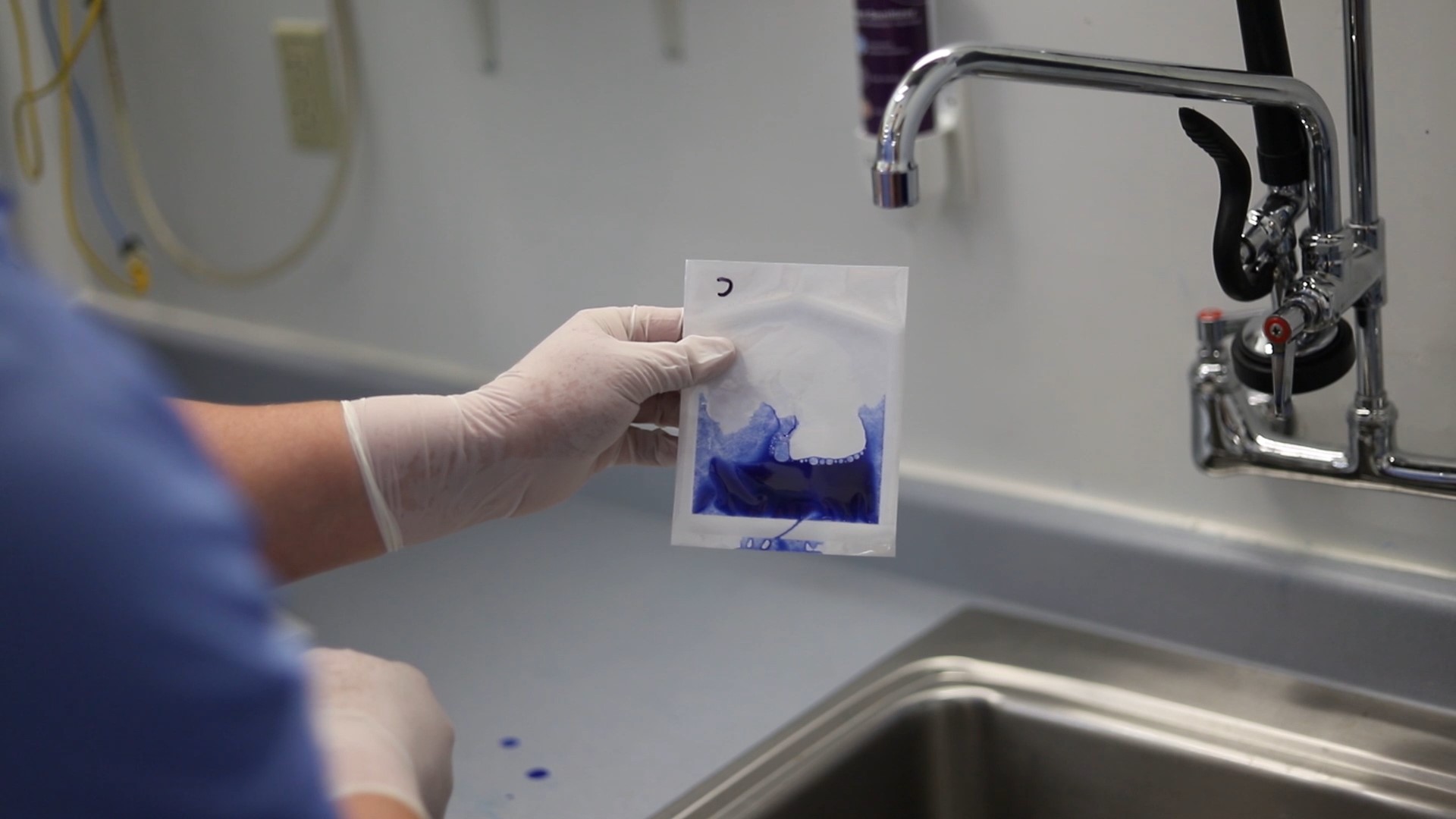
Dye testing is great for testing a sealing process output for integrity. You can tell whether or not your sealing process is making fully-formed seals.
3) Bubble Leak Testing
With bubble leak testing, you will first create a small hole in your package, attach your testing equipment over the hole, and then raise the equipment to a set pressure determined by a positive control. You will then submerge the package underwater and check for any leaks. If there is a leak, you will see a stream of bubbles floating from the package.

Bubble leak testing is great for entire package testing. If you do a package validation and you’d like to see if you may have developed a pinhole leak during your distribution simulation, this is an excellent test method to determine if you have integral sterile barriers.
Does your device packaging meet the medical device industry’s extensive requirements? QTS completes rigorous package and seal validations to ensure that every package meets the requirements to protect medical devices by complying with ISO and ASTM standards.
Contact us to learn more about how QTS can assist with your medical device packaging projects.



