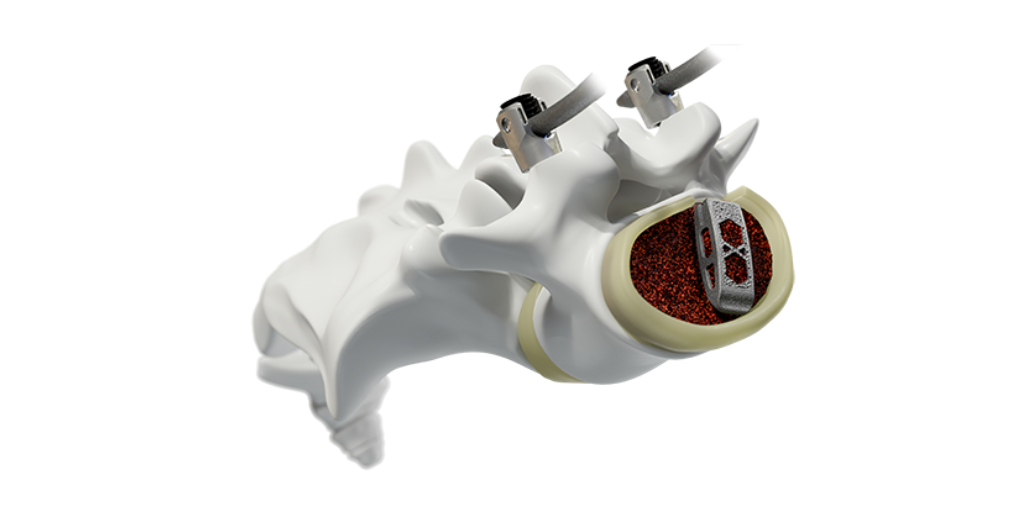
Medtronic Launches Proprietary Surface Technology on AM Devices Medtronic became interested in additive manufacturing (AM) to extend its innovation, unlocking advantages exclusive to the technology..

Medtronic Launches Proprietary Surface Technology on AM Devices Medtronic became interested in additive manufacturing (AM) to extend its innovation, unlocking advantages exclusive to the technology..

Cretex Medical | CDT Company, JunoPacific, Announces Operations Consolidation JunoPacific is in the process of combining its Redwood City and Soquel operations into a single site in Soquel, CA.

QTS receives Global First in Sterile Device Packaging from MedAccred Cretex Medical | QTS, a leader in medical device outsourcing solutions, has received accreditation from MedAccred® as the Global.

Meier Celebrates 40 Years As Meier celebrates its 40th-anniversary milestone, its focus—as always—is on its customers as it continues to grow, innovate and invest to meet their ever-expanding needs..

rms to Expand its Coon Rapids Facility rms has announced that it will be adding 60,000 sq. feet to its Coon Rapids manufacturing facility with construction beginning in 2019.

Expanding to Meet Your Needs: QTS Opens New Warehouse QTS is pleased to announce the consolidation of our warehouse operations into a single, new location. August 26, 2018 Cretex Medical | QTS.

Cretex Companies Acquires Leading Medical Packaging Services Provider QTS is widely recognized as a leading provider of validated assembly, kitting, and packaging solutions. June 13, 2017 Cretex.

Compliance to the UDI regulation includes the FDA public database, GUDID. The first phase of the Global Unique Device Identification Database (GUDID): Guidance for Industry document is now available..

DuPont Tyvek Transition Update DuPont is transitioning Tyvek® 1073B and Tyvek® 1059B to manufacturing lines that use the latest flash-spinning technology to help ensure greater continuity and.