
New Sterility Assurance Strategies for Products Sensitive to Sterilization Processes QTS was featured in AAMI ARRAY by Sopheak Srun & Molly Swanson | October 26, 2021 Sopheak Srun, MPH, SM(NRCM),.

New Sterility Assurance Strategies for Products Sensitive to Sterilization Processes QTS was featured in AAMI ARRAY by Sopheak Srun & Molly Swanson | October 26, 2021 Sopheak Srun, MPH, SM(NRCM),.

Cleaning Considerations for Additively Manufactured Parts Cretex Medical | QTS was recently featured in BONEZONE magazine to discuss additive manufacturing.

Women in Medtech 2021: Angie Hillyard, Spectralytics Engineering Director by Sponsored Content, Medical Device & Outsourcing | October 12, 2021 Angie Hillyard, Engineering Director at Cretex Medical,.
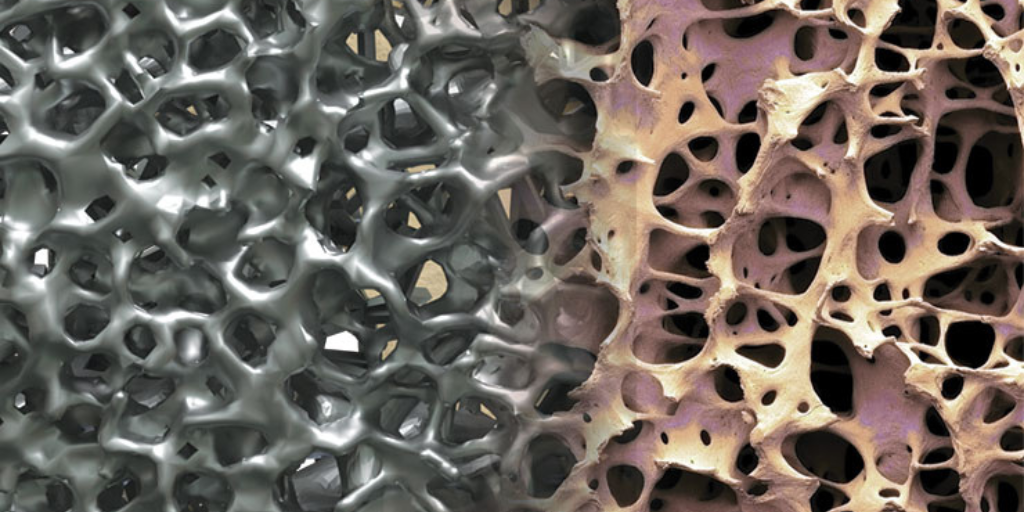
Metal Injection Molding and Metal 3D Printing Examination Both MIM and metal AM serve as alternative options to machining for medtech manufacturers requiring metal components.
QTS Collaborates with Other Industry Experts to Publish Best Practices Article on Tests of Sterility for EO Sterilization Sopheak Srun, a principal sterilization specialist at QTS, recently worked.

Sopheak Srun Appointed Co-Chair of an AAMI Working Group Sopheak, QTS Principal Sterilization Specialist, will be contributing to new and existing ISO standards to be upheld internationally.

Cretex Companies Announces Plans for New Facility Cretex Companies have signed a purchase agreement for land in Brooklyn Park, MN for a brand new facility.

From Powder to Package: Identifying the Right AM Suppliers Medical device contract manufacturer rms Company watched additive manufacturing develop and evolve, and ultimately saw an opportunity to.

Cretex Companies Announces New CFO Cretex Companies has announced the retirement of CFO Steve Ragaller.

Cretex Companies Announces Leadership Transition Cretex Companies has announced the retirement of CEO Lynn Schuler