.png)
Cretex Medical Announces Plans for Costa Rica Facility Cretex Medical announced today that they have broken ground on a new production facility in Cartago, Costa Rica. The 65,000-square-foot medical.
.png)
Cretex Medical Announces Plans for Costa Rica Facility Cretex Medical announced today that they have broken ground on a new production facility in Cartago, Costa Rica. The 65,000-square-foot medical.

Navigating Current Challenges with Sterilization Capacity Constraints In recent years, the medical device industry has faced growing sterilization capacity constraints. The primary causes are.

Technology Trends for Structural Heart Solutions — A Medtech Makers Q&A Replacing heart valves has become a more successful medical procedure thanks to the devices used to perform it. by Sean Fenske,.

Furthering Orthopedic Implant Fabrication Methods Developers and manufacturers of implants have added new tools to their belts to create orthopedic technologies using innovative techniques. by Mark.

Cretex Companies, Inc. Plastic Recycling Program Secures Bench for Local Elementary School In 2023, Cretex employees at the Brooklyn Park facility began participating in a program that recycled film.
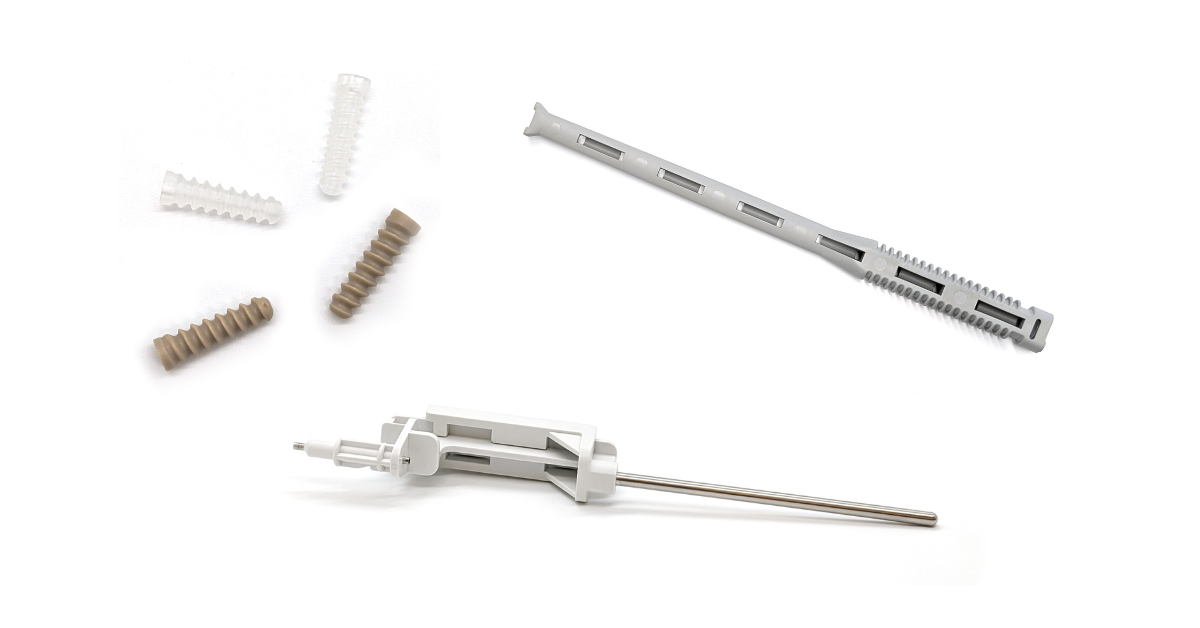
Molding Is Taking Its Place in Orthopedic Device Manufacturing As molders continue to expand capabilities attractive to orthopedic device makers, the fabrication process is growing in use. by Mark.

Abbott Electrophysiology Leaders Highlight Current Portfolio, Give Glimpse of Potential PFA Device In the latest episode of AbbottTalks, Abbott Cardiovascular’s leaders, Christopher Piorkowski, MD,.

QTS Renovation: A Major Transformation for Future Success In 2023, Cretex Medical | Quality Tech Services (QTS) embarked on a remarkable journey of transformation with the completion of a.
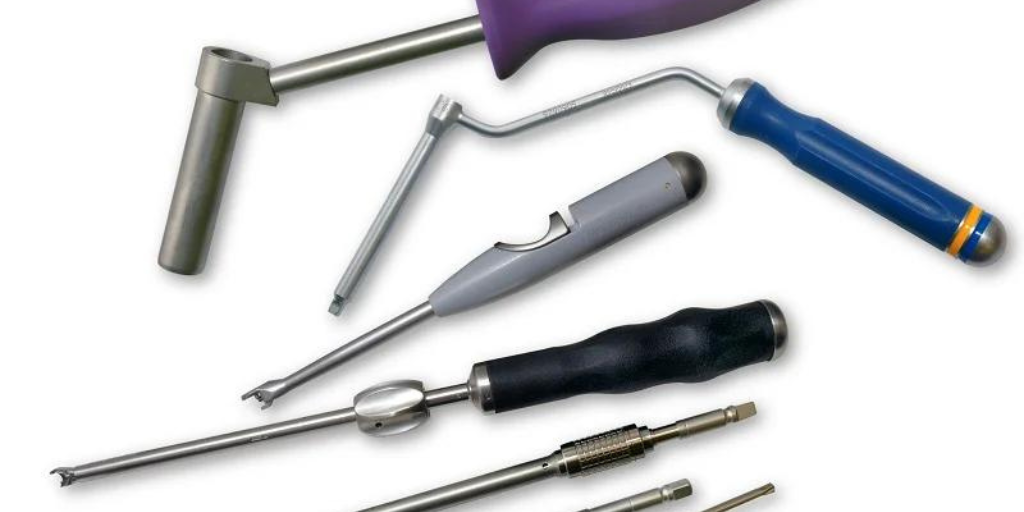
Cretex Medical’s Approach to Surgical Instrumentation Featured in Orthopedic Design & Technology (ODT) Magazine Granger Lubich, Engineering Manager for Cretex Medical | rms was featured in Orthopedic.
Overkilling Uncertainty, New TIR Seeks Clarity for Interpreting Bioburden Data When companies are testing a medical device’s bioburden — a measure of how much and what types of viable organisms are.