Cleaning Considerations for Additively Manufactured Parts
Cretex Medical | QTS was recently featured in BONEZONE magazine to discuss additive manufacturing.
by Cretex Medical | QTS | October 13, 2021
Cretex Medical | QTS was featured in BONEZONE Magazine.
To date, cleaning processes in the medical device industry have been primarily focused on devices made with traditional manufacturing practices such as milling and Swiss turning. Implants once produced via subtractive manufacturing are increasingly being produced with additive manufacturing (AM) because of its ability to produce complex geometries and intricate lattice structures. These features have many benefits such as facilitating bone ingrowth, integration and customization. However, as the intricacy of AM parts increases, so do the challenges in cleaning these parts.
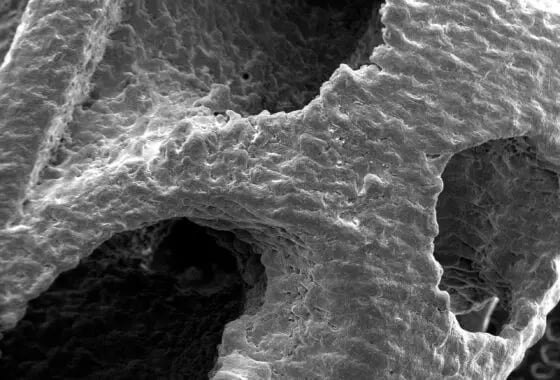
AM parts can be especially difficult to clean because they have a combination of lattice geometries, critical rough surfaces and machined details. Removing residual AM powder and machining fluids from all surfaces can be complicated.
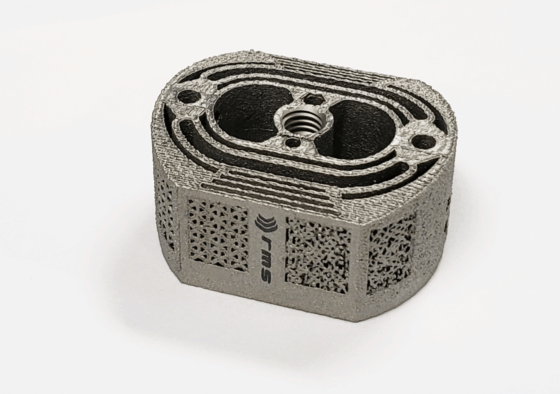
Complex Geometries
One of the most exciting developments with AM is the ability to create complicated geometries that subtractive machining is unable to produce. The drawback is that these composite shapes have increased surface area and create cavities that are hard to reach with traditional, ultrasonic cleaning solutions. These cavities also make it difficult to adequately rinse residue from AM parts.
Particulate removal is an important consideration when cleaning additive parts with complex geometries. With laser powder bed fusion (LPBF) technology, powdered metal (e.g., titanium) is melted to create the part. At the end of the manufacturing process, all un-melted particulates must be removed from the part, as excess powder can lead to patient complications.
According to the ASTM guidance document F33325-20, testing and validating the removal of manufacturing material residue should account for worst-case conditions such as areas with the “greatest amount of residual manufacturing materials, and a combination of largest surface area, greatest porosity, and most internal voids for sterilization validation” (26). In order to accurately validate the cleaning process and evaluate a complex part gravimetrically, a sample part may need to be designed in a way that allows it to be disassembled so difficult-to-reach areas can be exposed for testing. The part could also be created by welding two separate pieces together and breaking them apart again for testing.
Ultra-sonic cleaning is the most efficient way to clean many components. However, with AM parts, conventional ultra-sonic cleaning may not be sufficient. Using this method, a complex part may have to be physically turned in different directions to ensure that all particulates are extracted. Due to these challenges, alternative methods, such as vapor degreasing, are becoming more common. These new cleaning processes can be validated to be low residue and to meet rigorous standards.
Critical Surfaces
When using the ultra-sonic cleaning method, it is best to put the part directly in a metal basket to allow the ultra-sonic waves to do their work. However, placing metal AM parts directly on metal cleaning baskets could damage or scratch the surface of the part. Placing polymers in the cleaning basket would provide a buffer between the AM part and the basket, but the polymer would absorb the agitation and diminish the cleaning capability. So, for AM parts, it is preferred to use a basket with a coating that will protect the surface finish, but still allow the ultra-sonic waves to properly clean the part. Finding the balance between cushion for the part and compatibility with the cleaning process can be challenging.

Multiple Manufacturing Methods
Components produced solely through AM have specific cleaning processes. Most parts created with only AM can be cleaned with polar solutions, as they do not come in contact with oil or non-polar based residues during the manufacturing process. When a part is produced via a combination of additive and subtractive manufacturing, the cleaning process becomes more complex.
As stated in ASTM F33325-20, additive manufacturing “allows porous structures to be produced earlier in the manufacturing process…which can result in greater soiling by the manufacturing material of those porous structures throughout the rest of the process” (26). For example, if a part is initially printed with AM and then milled to create threads, it will be subjected to cutting fluids that will soil the porous structure of the part. The challenge then arises of getting the non-polar lubricants off of complex AM parts because they may not be able to be cleaned with the same method as a traditional subtractive manufactured component. For parts produced with multiple manufacturing methods, there are often different material and physical specifications which are compounded by factors like complex geometries and critical surface finishes.
Customized Cleaning Processes
There is no standard cleaning process that will work for every AM product. Each part has different features, surface finishes, requirements and limitations, and consequently, requires its own unique cleaning process. Matt Homuth, Senior Validation Engineer at QTS, said, “Cleaning processes need to be adapted specifically to work with the complexity and surface texture of AM parts. Steps considered to be routine, like ultra-sonic cleaning, may need to be adjusted because of complex geometries and critical surface finishes.” Experienced sterilization specialists know what is required to adequately clean AM parts and can customize cleaning processes to meet the needs of each product.
The medical device industry continues to produce increasingly intricate AM parts that are posing more complex cleaning challenges. Cleaning processes for medical devices are adapting in response to these challenges by incorporating novel techniques and processes. It is important to partner with a contract manufacturer that is skilled in sterilization management and can handle validating new and unique cleaning processes.
QTS provides contract services including critical final cleaning of components and devices, as well as finished device assembly & kitting, sterile packaging and sterilization management. Certified staff microbiologists and sterilization specialists oversee the process, and provide complete validation services including creating protocols, sample preparation, and coordinating third-party laboratory testing. The company customizes their processes to meet the specifications of each product and the regulatory requirements for the device to be certified sterile.
This article originally appeared in BONEZONE Magazine.