
Cretex Medical Exhibits at MD&M West 2026 Cretex Medical traveled to Anaheim, California to attend and exhibit at MD&M West 2026, one of the largest multi-sector manufacturing trade shows in the.

Cretex Medical Exhibits at MD&M West 2026 Cretex Medical traveled to Anaheim, California to attend and exhibit at MD&M West 2026, one of the largest multi-sector manufacturing trade shows in the.

Cretex Medical Signs Agreement to Acquire Atemisa Precision, S.A. in Costa Rica Cretex Medical, a leading provider of contract medical device manufacturing and engineering services, announced today.

Cretex Medical Sponsors Thumbs Up Annual 5K/10K Walk/Run/Bike Event

Cretex Companies Rallies for Communities in Annual Cares-A-Thon Each June, our teams across the country unite for the Cretex Cares-A-Thon, a month-long celebration of service, generosity, and.

Cretex Medical | rms Celebrates Production of One Millionth Additive Manufactured Part Cretex Medical | rms recently celebrated the production of its One Millionth Additive Manufactured Part, a major.

Cretex Medical | rms Receives Minnesota Governor’s Workplace Safety Silver Award Cretex Medical | rms is proud to announce that we were honored with the Minnesota Governor’s Workplace Safety Silver.
.png)
Cretex Medical | CDT Brooklyn Park Teams Raise $6,950 for Local Charities At Cretex Medical, giving back is a team effort, and our Brooklyn Park employees took that spirit to the next level this.

Cretex Medical | rms Team Member Ryan Kircher Honored with Distinguished Innovator Operators Award at AMUG 2025 Cretex Medical proudly celebrates Ryan Kircher, Principal Manufacturing Engineer at.

Cretex Medical’s Advanced Instrument Capabilities Featured in Orthopedic Design & Technology (ODT) Magazine Korey Lieser, Instruments Operations Manager at Cretex Medical | rms, was featured in.
.png)
Cretex Medical | rms Volunteers at The Dwelling Place for Domestic Violence Awareness Month October is Domestic Violence Awareness Month - a time to shine a light on the struggles faced by survivors.

Additive Manufacturing: Moving Beyond Implants - An Orthopedic Innovators Q&A With additive manufacturing becoming more of a production process, it’s critical to find the right suppliers who have.

Cretex Medical | rms Donates Blankets to the Elderly Cretex Medical | rms employees from Anoka and Coon Rapids crafted 12 blankets and over a dozen cards for elderly residents, with volunteer.

Cretex Medical Shows Continuous Support by Participating in Thumbs Up’s 11th Annual 5K & 10K Run/Walk/Bike As a long-time supporter of Thumbs Up, Cretex Medical showed up with a strong team of.

Corporate Social Responsibility (CSR) at Cretex Companies At Cretex, our values are the foundation of everything we do. While times may evolve and new challenges emerge, our unwavering commitment to.
Medical Device Machinists Are Multi-Task Masters Medtech machining equipment has evolved to meet customers’ demands for micro components, material variability, more complex shapes, and tighter.
Women in MedTech: Rona Gungon, Product Development Engineering Supervisor at Cretex Medical Rona Gungon is a Product Development Engineering Supervisor with over 17 years of experience in the medical.

Cretex Medical Employees Unite for the Community: A Successful Backpack Drive Cretex Medical recently hosted an Adopt-A-Backpack Backpack Drive for the Anoka-Hennepin School District.

A Sunny Day of Fun and Giving Back: Cretex Companies' Annual Golf Tournament Thanks to everyone's generosity, we raised $115 and donated 367 pounds of food to the ACBC Food Shelf, making a meaningful.

Unlock the Potential: Elevate Your Manufacturing with 3D Printing The landscape of healthcare manufacturing is evolving at an unprecedented pace, thanks in part to the impact of additive.
.png)
Cretex Medical Announces Plans for Costa Rica Facility Cretex Medical announced today that they have broken ground on a new production facility in Cartago, Costa Rica. The 65,000-square-foot medical.

Navigating Current Challenges with Sterilization Capacity Constraints In recent years, the medical device industry has faced growing sterilization capacity constraints. The primary causes are.

Technology Trends for Structural Heart Solutions — A Medtech Makers Q&A Replacing heart valves has become a more successful medical procedure thanks to the devices used to perform it. by Sean Fenske,.

Furthering Orthopedic Implant Fabrication Methods Developers and manufacturers of implants have added new tools to their belts to create orthopedic technologies using innovative techniques. by Mark.

Cretex Companies, Inc. Plastic Recycling Program Secures Bench for Local Elementary School In 2023, Cretex employees at the Brooklyn Park facility began participating in a program that recycled film.
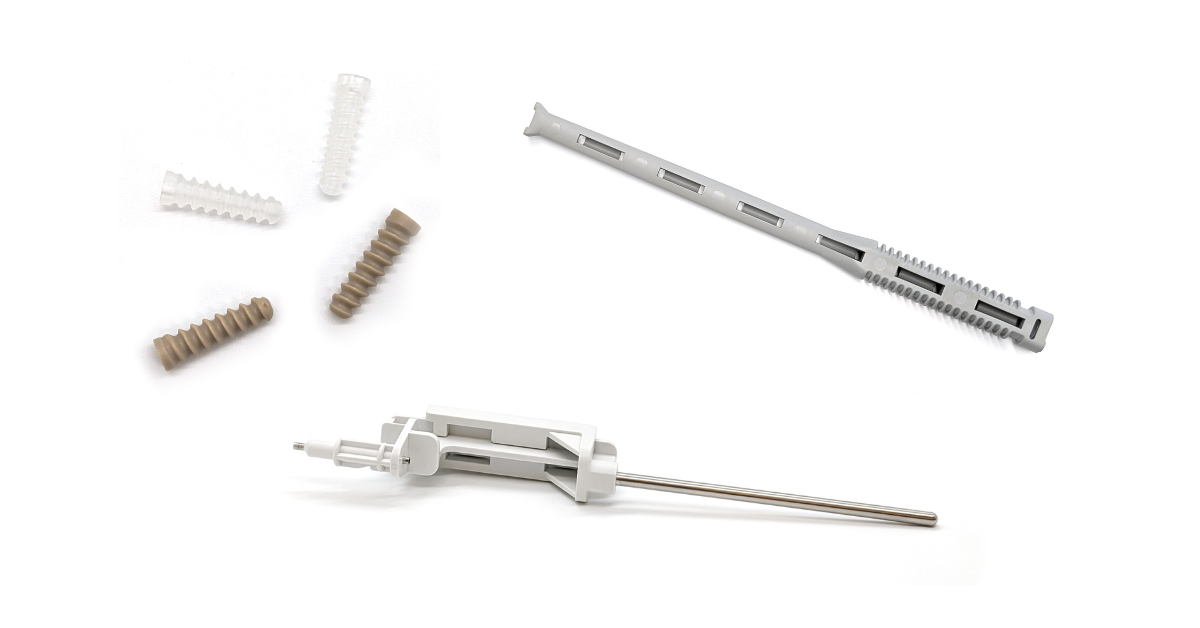
Molding Is Taking Its Place in Orthopedic Device Manufacturing As molders continue to expand capabilities attractive to orthopedic device makers, the fabrication process is growing in use. by Mark.

Abbott Electrophysiology Leaders Highlight Current Portfolio, Give Glimpse of Potential PFA Device In the latest episode of AbbottTalks, Abbott Cardiovascular’s leaders, Christopher Piorkowski, MD,.

QTS Renovation: A Major Transformation for Future Success In 2023, Cretex Medical | Quality Tech Services (QTS) embarked on a remarkable journey of transformation with the completion of a.
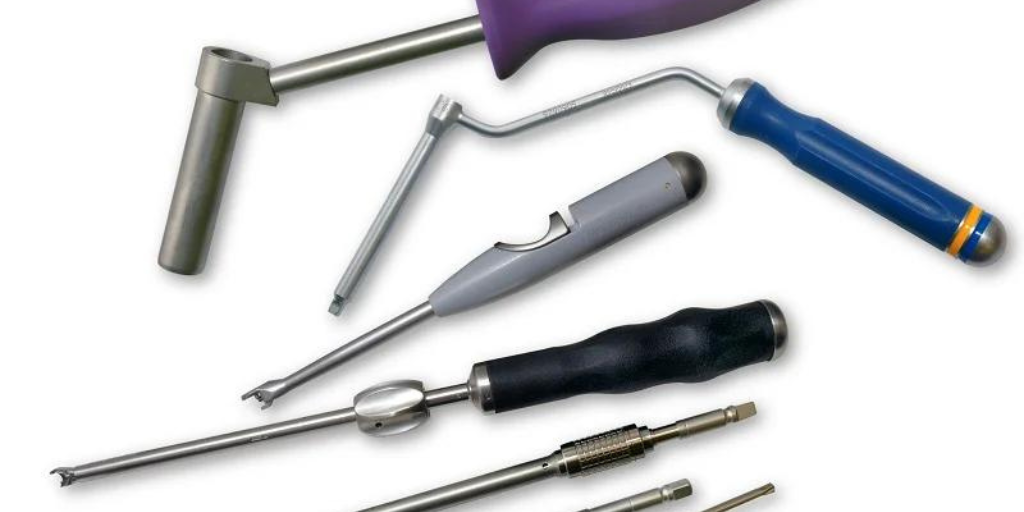
Cretex Medical’s Approach to Surgical Instrumentation Featured in Orthopedic Design & Technology (ODT) Magazine Granger Lubich, Engineering Manager for Cretex Medical | rms was featured in Orthopedic.
Overkilling Uncertainty, New TIR Seeks Clarity for Interpreting Bioburden Data When companies are testing a medical device’s bioburden — a measure of how much and what types of viable organisms are.

Leveraging Automation to Improve Inspection Quality—An Orthopedic Innovators Q&A Automation not only addresses labor shortage concerns, but also enhances the overall output by improving the.

Women in Medtech: Lauren Roy, Engineering Supervisor, Cretex Medical Lauren Roy began her career at Cretex Medical as a Quality Intern and her dedication and performance led to a full-time role. Over.

Considerations for Bringing Neurostim Innovations to Market—A Medtech Makers Q&A As alternatives to pharmaceuticals, neuro devices can provide benefits without the side effects for those who require.

Sharing Our Additive Manufacturing Expertise with Tech Briefs Readers Additive manufacturing experts at rms recently contributed to an article featured in Tech Briefs’ Special Report: Medical.

Women in Medtech: Janelle Swanson, Business Unit Director at QTS, a Cretex Medical Company by Sponsored Content, Medical Device & Outsourcing | October 3, 2022 Janelle Swanson, Business Unit Director.

Medtech Manufacturing Cleaning and Sterilization Validation—A Medtech Makers Q&A Ensuring components remain residue-free and are properly sterilized are critical tasks for medical device firms. by.

How Industrial CT Scanning Improves Additive Manufacturing OEMs are seeking manufacturing partners who can leverage multiple technologies to design and fabricate the instruments for orthopedic.

Spectralytics - Impacting Lives Daily Spectralytics has been a pioneer in laser technology for the medical device space for over twenty years, paving the way for current industry standards. by Laure.

AM for Production: Where Conventional Lessons Do and Do Not Apply As RMS has grown its additive manufacturing division, it has discovered which principles of subtractive manufacturing apply to.

New Sterility Assurance Strategies for Products Sensitive to Sterilization Processes QTS was featured in AAMI ARRAY by Sopheak Srun & Molly Swanson | October 26, 2021 Sopheak Srun, MPH, SM(NRCM),.

Cleaning Considerations for Additively Manufactured Parts Cretex Medical | QTS was recently featured in BONEZONE magazine to discuss additive manufacturing.

Women in Medtech 2021: Angie Hillyard, Spectralytics Engineering Director by Sponsored Content, Medical Device & Outsourcing | October 12, 2021 Angie Hillyard, Engineering Director at Cretex Medical,.
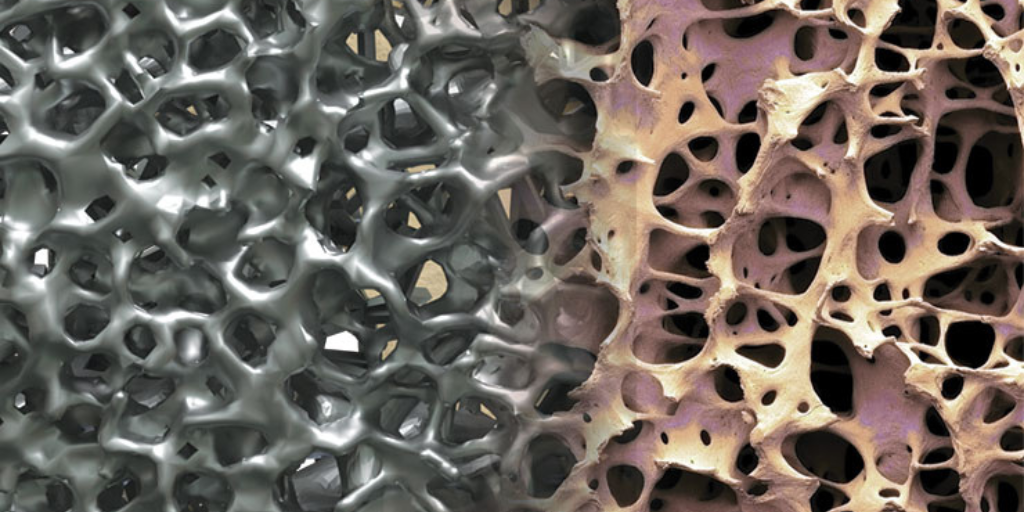
Metal Injection Molding and Metal 3D Printing Examination Both MIM and metal AM serve as alternative options to machining for medtech manufacturers requiring metal components.
QTS Collaborates with Other Industry Experts to Publish Best Practices Article on Tests of Sterility for EO Sterilization Sopheak Srun, a principal sterilization specialist at QTS, recently worked.

Sopheak Srun Appointed Co-Chair of an AAMI Working Group Sopheak, QTS Principal Sterilization Specialist, will be contributing to new and existing ISO standards to be upheld internationally.

Cretex Companies Announces Plans for New Facility Cretex Companies have signed a purchase agreement for land in Brooklyn Park, MN for a brand new facility.

From Powder to Package: Identifying the Right AM Suppliers Medical device contract manufacturer rms Company watched additive manufacturing develop and evolve, and ultimately saw an opportunity to.

Cretex Companies Announces New CFO Cretex Companies has announced the retirement of CFO Steve Ragaller.

Cretex Companies Announces Leadership Transition Cretex Companies has announced the retirement of CEO Lynn Schuler
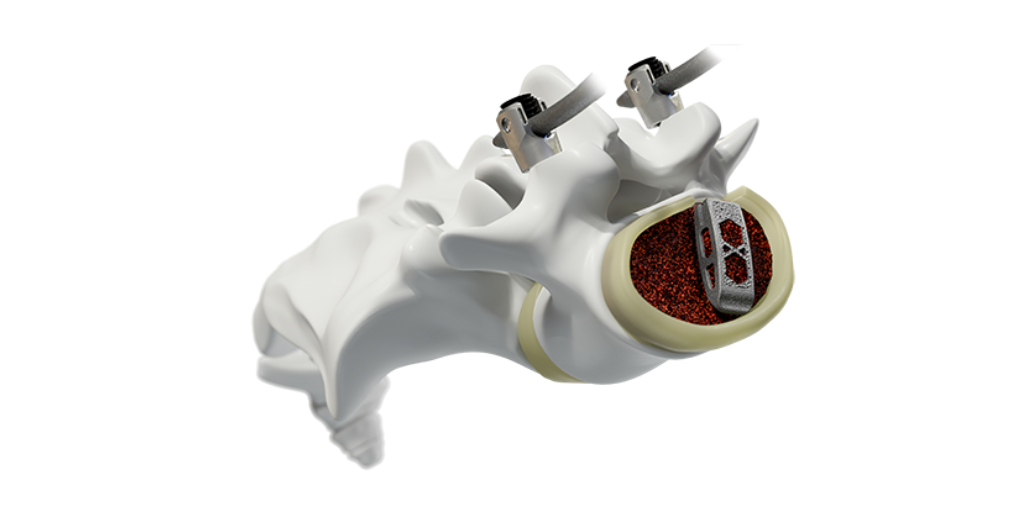
Medtronic Launches Proprietary Surface Technology on AM Devices Medtronic became interested in additive manufacturing (AM) to extend its innovation, unlocking advantages exclusive to the technology..

Cretex Medical | CDT Company, JunoPacific, Announces Operations Consolidation JunoPacific is in the process of combining its Redwood City and Soquel operations into a single site in Soquel, CA.

QTS receives Global First in Sterile Device Packaging from MedAccred Cretex Medical | QTS, a leader in medical device outsourcing solutions, has received accreditation from MedAccred® as the Global.

Meier Celebrates 40 Years As Meier celebrates its 40th-anniversary milestone, its focus—as always—is on its customers as it continues to grow, innovate and invest to meet their ever-expanding needs..

rms to Expand its Coon Rapids Facility rms has announced that it will be adding 60,000 sq. feet to its Coon Rapids manufacturing facility with construction beginning in 2019.

Expanding to Meet Your Needs: QTS Opens New Warehouse QTS is pleased to announce the consolidation of our warehouse operations into a single, new location. August 26, 2018 Cretex Medical | QTS.

Cretex Companies Acquires Leading Medical Packaging Services Provider QTS is widely recognized as a leading provider of validated assembly, kitting, and packaging solutions. June 13, 2017 Cretex.

Compliance to the UDI regulation includes the FDA public database, GUDID. The first phase of the Global Unique Device Identification Database (GUDID): Guidance for Industry document is now available..

DuPont Tyvek Transition Update DuPont is transitioning Tyvek® 1073B and Tyvek® 1059B to manufacturing lines that use the latest flash-spinning technology to help ensure greater continuity and.

Unique Device Identification (UDI) Final Rule was announced by the FDA on September 20, 2013. The rule was published in the Code of Federal Regulations as of September 24, 2013. Implementation for.