
Medtech Manufacturing Cleaning and Sterilization Validation—A Medtech Makers Q&A Ensuring components remain residue-free and are properly sterilized are critical tasks for medical device firms. by.

Medtech Manufacturing Cleaning and Sterilization Validation—A Medtech Makers Q&A Ensuring components remain residue-free and are properly sterilized are critical tasks for medical device firms. by.

How Industrial CT Scanning Improves Additive Manufacturing OEMs are seeking manufacturing partners who can leverage multiple technologies to design and fabricate the instruments for orthopedic.

Women in Medtech 2021: Angie Hillyard, Spectralytics Engineering Director by Sponsored Content, Medical Device & Outsourcing | October 12, 2021 Angie Hillyard, Engineering Director at Cretex Medical,.

From Powder to Package: Identifying the Right AM Suppliers Medical device contract manufacturer rms Company watched additive manufacturing develop and evolve, and ultimately saw an opportunity to.
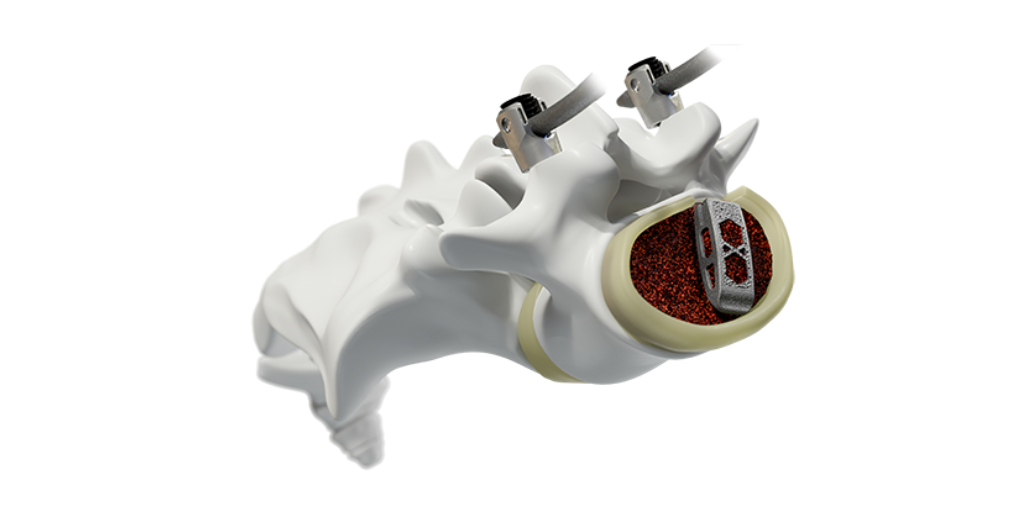
Medtronic Launches Proprietary Surface Technology on AM Devices Medtronic became interested in additive manufacturing (AM) to extend its innovation, unlocking advantages exclusive to the technology..

Compliance to the UDI regulation includes the FDA public database, GUDID. The first phase of the Global Unique Device Identification Database (GUDID): Guidance for Industry document is now available..

DuPont Tyvek Transition Update DuPont is transitioning Tyvek® 1073B and Tyvek® 1059B to manufacturing lines that use the latest flash-spinning technology to help ensure greater continuity and.

Unique Device Identification (UDI) Final Rule was announced by the FDA on September 20, 2013. The rule was published in the Code of Federal Regulations as of September 24, 2013. Implementation for.