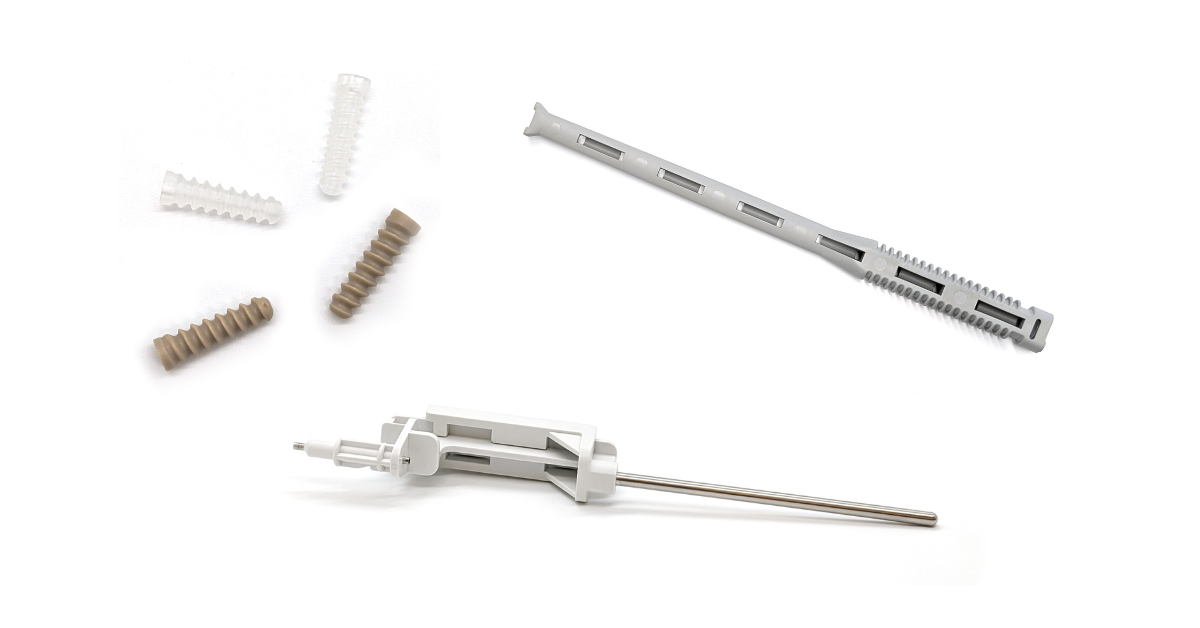
Molding Is Taking Its Place in Orthopedic Device Manufacturing As molders continue to expand capabilities attractive to orthopedic device makers, the fabrication process is growing in use. by Mark.

Molding Is Taking Its Place in Orthopedic Device Manufacturing As molders continue to expand capabilities attractive to orthopedic device makers, the fabrication process is growing in use. by Mark.

Abbott Electrophysiology Leaders Highlight Current Portfolio, Give Glimpse of Potential PFA Device In the latest episode of AbbottTalks, Abbott Cardiovascular’s leaders, Christopher Piorkowski, MD,.

QTS Renovation: A Major Transformation for Future Success In 2023, Cretex Medical | Quality Tech Services (QTS) embarked on a remarkable journey of transformation with the completion of a.
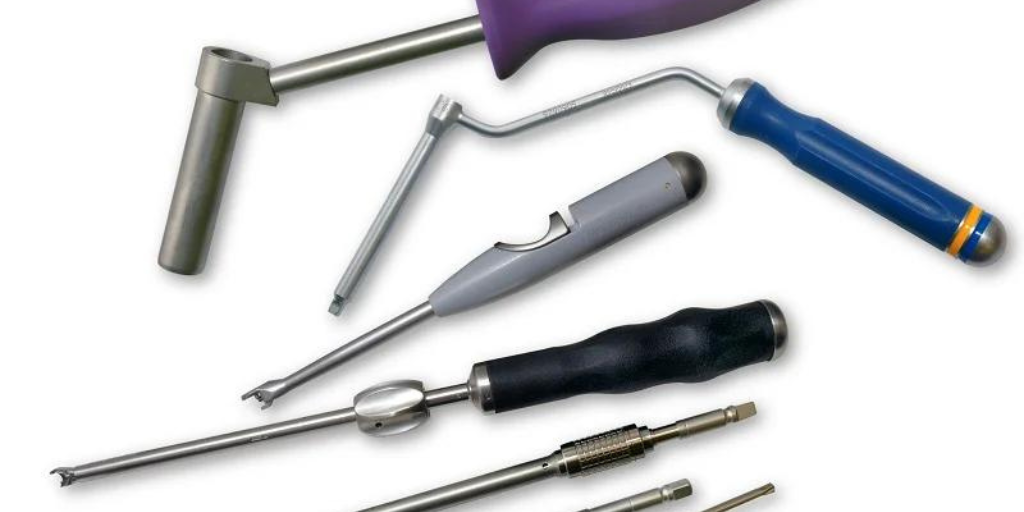
Cretex Medical’s Approach to Surgical Instrumentation Featured in Orthopedic Design & Technology (ODT) Magazine Granger Lubich, Engineering Manager for Cretex Medical | rms was featured in Orthopedic.
Overkilling Uncertainty, New TIR Seeks Clarity for Interpreting Bioburden Data When companies are testing a medical device’s bioburden — a measure of how much and what types of viable organisms are.

Leveraging Automation to Improve Inspection Quality—An Orthopedic Innovators Q&A Automation not only addresses labor shortage concerns, but also enhances the overall output by improving the.

Women in Medtech: Lauren Roy, Engineering Supervisor, Cretex Medical Lauren Roy began her career at Cretex Medical as a Quality Intern and her dedication and performance led to a full-time role. Over.

Considerations for Bringing Neurostim Innovations to Market—A Medtech Makers Q&A As alternatives to pharmaceuticals, neuro devices can provide benefits without the side effects for those who require.

Sharing Our Additive Manufacturing Expertise with Tech Briefs Readers Additive manufacturing experts at rms recently contributed to an article featured in Tech Briefs’ Special Report: Medical.

Women in Medtech: Janelle Swanson, Business Unit Director at QTS, a Cretex Medical Company by Sponsored Content, Medical Device & Outsourcing | October 3, 2022 Janelle Swanson, Business Unit Director.