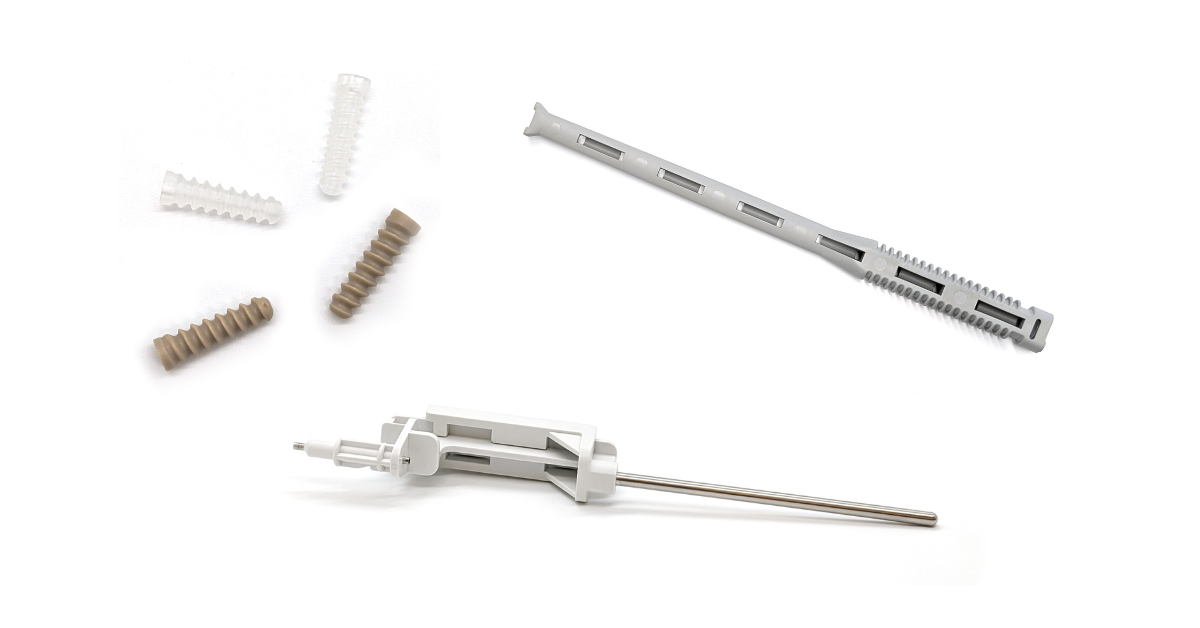
Considerations for Bringing Neurostim Innovations to Market—A Medtech Makers Q&A
As alternatives to pharmaceuticals, neuro devices can provide benefits without the side effects for those who require them.
by Sean Fenske, MPO Editor-in-Chief | August 28, 2023
Jim Biggs, Director of NPI Technical Solutions at Cretex Medical, was featured in Medical Product Outsourcing magazine.
Pharmaceuticals have been a primary therapy for a number of neurological conditions for quite some time. Unfortunately, there are a number of factors that make this treatment option less than ideal, such as patient non-compliance and numerous negative side effects. As such, an alternative to “popping pills” has been sought after for some time.
Fortunately, the medical device industry has been developing a substitute to pharmaceuticals for those suffering from conditions such as chronic pain, depression, epilepsy, and other conditions. As more companies begin to work on these technologies, it is important for them to ensure they are aware of development and manufacturing issues that can stifle a successful market launch.
Sharing some of the factors medical device organizations need to keep top of mind when planning a neurostimulation device is Jim Biggs, director of NPI Technical Solutions at Cretex Medical. In the following Q&A, he outlines considerations developers should plan for while also revealing the benefits that can be realized by working with the right manufacturing partner.
Sean Fenske: Neurostim devices have been gaining momentum in healthcare. Before we tackle the considerations, can you first speak about what’s driving the market toward these technologies?
Jim Biggs: The neurostimulation device market has been growing exponentially for the past several years. Significant growth is being driven by a need to develop safe and non-invasive alternatives with little to no patient side effects that neuro devices can offer compared to pharmaceutical drugs, which may have harmful side effects for the patient. In most cases, these neuro device therapies are much more effective and target the specific anatomy, which greatly reduces collateral anatomical harm to other areas of the human body.
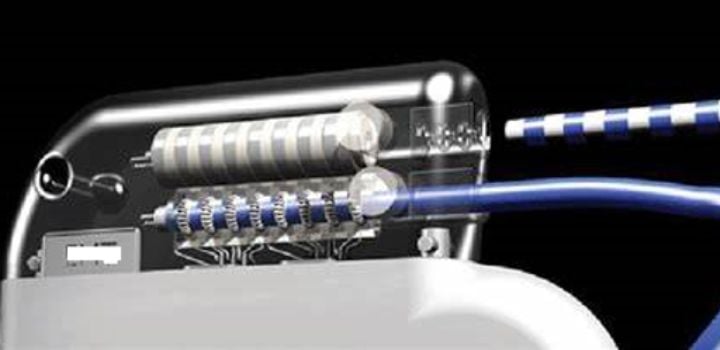 The global neuromodulation market is seeing a larger demand for these therapies for the treatment of chronic pain, Parkinson’s disease, depression, epilepsy, and other neurological conditions which makes these alternatives very attractive world-wide.
The global neuromodulation market is seeing a larger demand for these therapies for the treatment of chronic pain, Parkinson’s disease, depression, epilepsy, and other neurological conditions which makes these alternatives very attractive world-wide.
Fenske: When it comes to ideation around a new neurostim device, what factors must be worked out?
Biggs: When considering the ideation of a new neurostimulation device, several factors need to be addressed:
- Demand: Is there a strong demand for the specific medical indication the device aims to address?
- Patient Benefit: Does the device offer a valuable and beneficial alternative for patients?
- Patient Safety: Can the device ensure the safety of the patient during its use?
- Regulatory Compliance: Will the product meet the necessary regulatory requirements and standards?
- Surgical Procedure: What does the surgical procedure involving the device entail? Is it invasive or non-invasive?
- Reimbursement: Is the product likely to be reimbursable by healthcare systems or insurance providers?
- Manufacturability: Can the device be manufactured per the design and specifications?
- Materials: Are the required raw materials readily accessible for production?
- Cost Effectiveness: Does the device offer a cost-effective solution for OEMs and/or manufacturers? For patients and healthcare providers?
- Scalability: Can the product be efficiently scaled for high-production manufacturing?
- Make or Buy Decision: Should the components be produced in-house or purchased from external manufacturers/suppliers?
Addressing these factors ensures the success and viability of the new neurostimulation device.
Fenske: How much input does a supply partner like Cretex Medical have on the development of the device? What value does it bring to have the OEM and manufacturing engineers collaborate on the design?
Biggs: Generally, the OEM “owns” the design, however, Cretex Medical develops and implements the process design as it relates to the manufacturing of the product. We have demonstrated and implemented unique approaches as it relates to the manufacturing of neurostimulation products, which results in robust process designs while being very cost effective.
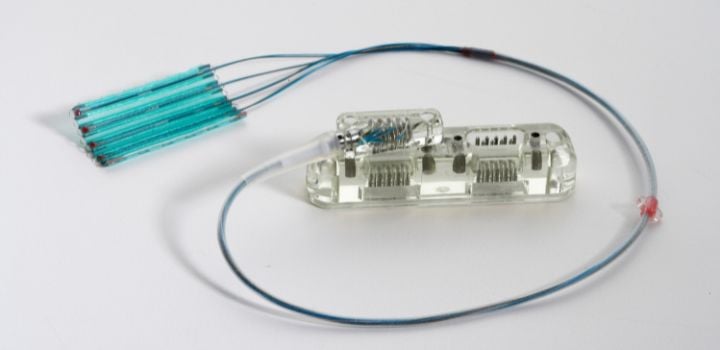
Cretex Medical also identifies the risks associated with the manufacturing of the product and addresses this upfront with the customer. There is a balance between meeting the product design intent and having the ability to manufacture the product, therefore, there is “give and take” on almost every program and the collaboration between the two engineering teams in the early phases is most critical.

Fenske: In terms of working with the supply partner, what are the most important factors to ensure a smooth project pathway to success?
Biggs: Ensuring the project schedule and milestones are accurate is one of the most important factors to ensure a successful project. OEMs base their testing, submissions, and approvals on the timelines and milestones set by their supplier partners. If these timelines are extended, the OEM is at risk of losing market share due to product launch delays. Therefore, it's crucial to establish an agreed-upon project plan and process validation approach prior to execution. A key component to maintaining the project schedule is resource availability of the project team (on both sides) throughout all phases of the project.
Properly designed tooling such as molds, dies, weld tooling, and inspection/assembly fixtures is equally important for ensuring a smooth project launch. The OEM’s approval of these designs is key, alongside the identification of tooling risks and potential solutions to mitigate them.
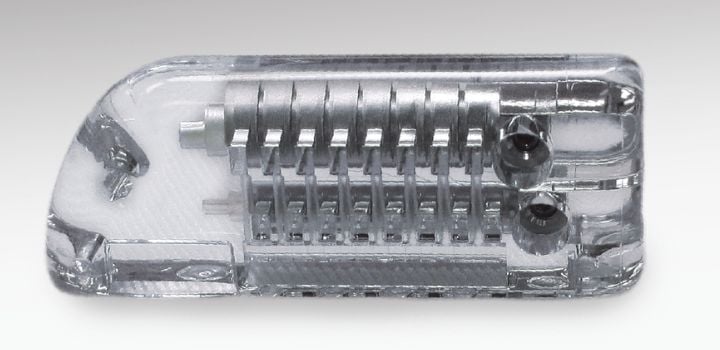
Early specification of all capital equipment and secondary operation equipment is necessary for the project's success. The Cretex Medical NPI Engineering group has dedicated Class 8 cleanrooms equipped with capital and secondary operations equipment. This setup ensures product development and validations are on track without interrupting production operations.
Biggs: We see project delays when teams are overly optimistic with their project timelines and overlook potential risks and delays that might arise. Unplanned changes in both product and tooling designs and multiple iterations of tooling adjustments can also impact the timeline.
During the process development phase, having complete information is preferable. When the team lacks evidence of the full process capability, it can lead to inadequate process development for Operational Qualification (OQ) and Performance Qualification (PQ) validations, potentially causing significant delays.
Additionally, delays within the supply chain and insufficient allocation of resources can further compound these challenges.